Upward Trend of ADHD Diagnoses
Attention deficit hyperactivity disorder (ADHD) has become the most commonly diagnosed childhood disorder in the United States. An estimated 6.4 million children ages 4 to 17 are diagnosed with ADHD at some point in their lives. This reflects a staggering 41% increase within the last decade. ADHD is characterized by patterns of inattention and hyperactive and impulsive behaviors that disrupt social, academic, and occupational functioning. ADHD was once viewed as a problem of discipline and poor parenting. However, a substantial body of empirical studies within the past couple of decades has established ADHD as a neuropsychiatric disorder with multiple biological abnormalities.
Conventional treatment for ADHD includes pharmacological or behavioral therapy, although the efficacy of medications in treating these conditions is widely debated. The majority of pharmaceutical agents used to treat ADHD include methylphenidate or amphetamine, which work by stimulating dopamine neurotransmission in the brain. However, stimulant medication is only effective for short-term symptom reduction, and only about 30% of clients with ADHD report any significant improvement in symptoms on stimulant medications.1 While some children may benefit from medications, it is equally imperative for parents, caregivers, and health care providers to consider complementary and integrative therapies to alleviate symptoms.
Behavioral therapies and neurofeedback have been proven to be effective in targeting abnormalities that underlie ADHD symptomatology. The American Academy of Pediatrics has designated neurofeedback as “Level 1 Best Support” – indicating that it is a safe, evidence-based treatment for childhood ADHD. Neurofeedback is a non-pharmacological intervention that has shown promise in the long-term management of ADHD symptoms by teaching individuals to adjust their brain wave activity through a reward system based on operant conditioning principles.
Several studies have demonstrated that individuals with ADHD have more theta brain wave activity and less beta brain wave activity.2 Theta brain waves are present during deep meditation and light sleep and have been negatively related to alertness. Beta brain waves are associated with normal waking consciousness, attentivity, and critical reasoning and are responsible for completing executive functioning tasks. Therefore, neurofeedback aims to increase levels of beta brain waves and reduce theta brain waves to increase cognitive and executive function in individuals with ADHD.
A recent study from researchers at Harvard Medical School found that children who participated in neurofeedback experienced improvements in ADHD symptoms that remained at the six-month follow-up.3 Children were randomly assigned to receive computer attention training using neurofeedback, cognitive training, or a control condition three times per week over five months for a total of 40 sessions. Based on parental reports on multiple different rating scales, those in the neurofeedback group showed significant improvements over time compared with the control condition on inattention, executive functioning, and hyperactivity/impulsivity. In addition to parent reports, the neurofeedback group had significant improvements on off-task motor/verbal changes in behavior. Changes occurred earlier and were stronger in the neurofeedback group than in the cognitive training group.
Neurofeedback has also been found to be as effective as medication in improving ADHD symptoms. In one study with 130 children, participants were randomized to receive only neurofeedback, neurofeedback with methylphenidate, or only methylphenidate.4 The group who received neurofeedback and methylphenidate experienced superior improvements in attentiveness overall, and the group who received only neurofeedback experienced effects that were equivalent to the medication-only group.
Similarly, a recent study compared the efficacy of neurofeedback in 131 students divided into four groups (neurofeedback group, pharmacological group, combined group, and no treatment group) and assessed participants’ executive control and cortical activation pre- and post- treatment.5 Compared to the other three groups after treatment, participants in the combined group showed the highest values of cortical activation, the best executive control, and the greatest reduction in hyperactivity and attention deficit symptoms.
Neurofeedback can be expensive and time-consuming, however; and in our experience, treatments that are cost-effective and simple to follow are the ones that maximize patient compliance. Plant-derived compounds known as oligomeric proanthocyanidins (OPCs) have proven be a promising adjuvant therapy in treating ADHD. Oligomeric proanthocyanidins are a form of polyphenols, compounds that plants produce as a defense against environmental harm. OPCs are typically present as plant pigments in cranberries, blueberries, and grapes, but they are also abundant in grapeseed, gingko biloba, plums, peaches, and pine bark. The ability of OPCs to stimulate activity in brain regions responsible for carrying out executive functioning tasks has been of growing interest among researchers seeking safe, natural approaches for the treatment of ADHD in children and adults.
Historical Uses of OPCs in Medicine
During the Age of Exploration, European explorers embarked on long, treacherous sea voyages toward North America. In 1534, the crew of French explorer Jacques Cartier were ship-bound for months, and many crew members succumbed to the devastating effects of scurvy.6 Cartier luckily befriended a tribe of Quebecois Native Americans during his explorations, who offered Cartier and his men a medicinal tea brewed from the needles and bark of special pine trees. This medicinal tea saved Cartier’s men, as documented in his journal, and has since fueled one of the most exciting breakthroughs in the field of nutritional psychiatry.
Long before Cartier and his men were introduced to the healing properties of the pine bark tea, OPC-rich plant extracts had already been used as medicine in China and India for millennia. Between 1100BC and 200BC, Chinese physicians ardently supported the drinking of green tea (Camellia sinensis) to maintain health; and by the Tang Dynasty (AD 618-907), tea had become an object of lay and medicinal veneration. Passages from ancient Indian texts also demonstrate a rich and lengthy history of using OPC-containing plants for therapeutic purposes: two plants that figure prominently in Ayurvedic literature (Cedrus deodara, or Himalayan cedar, and Pinus roxburghii, or Indian longleaf pine) have been described as possessing central nervous system effects, and have traditionally been used in Ayurvedic medicine to treat disorders of the mind.7
Multiple research studies have demonstrated that OPCs may directly benefit brain networks by enhancing communication between neurons and offer cell protection through their potent antioxidant defense. During over two decades of experience of utilizing OPCs in the treatment of ADHD patients, I have observed countless patients report improvement in their ability to concentrate, focus, and eliminate the need for medications.
OPCs for ADHD
As neuroimaging studies have confirmed the presence of atypical brain activity in ADHD individuals, targeted treatment efforts such as neurofeedback aim to improve brain wave activity. In our clinic, we have observed that supplementation with OPCs can dramatically improve ADHD symptomology. Over the years, EEG analyses have been utilized with many of our ADHD patients. We have recorded multiple cases demonstrating EEG changes, handwriting changes, enhanced academic performance, improved behavior, and improved CPT (continuance performance testing) after supplementation with OPCs. Prior to initiating OPC supplementation, we conducted baseline EEGs and compared baseline EEGs to EEGs following supplementation with OPCs.
Following OPC supplementation, patients reported subjective improvements in attention, focus, mood, and social interactions. We observed more equality in their theta:beta ratios such that theta waves are reduced significantly, particularly during the reading task (Figures 1.1 and 1.2). The EEG changes are very similar to what has been found with neurofeedback. However, neurofeedback can be expensive and time-consuming. While many individuals require a multi-modal treatment plan, treatment as simple as supplementation with OPCs can be efficacious and enhance patient compliance.
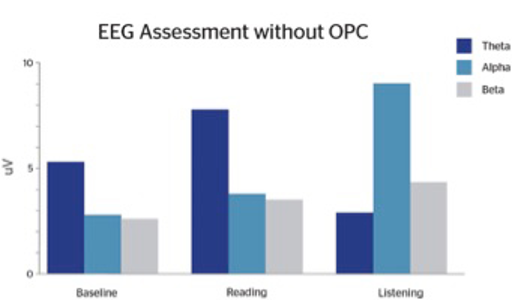
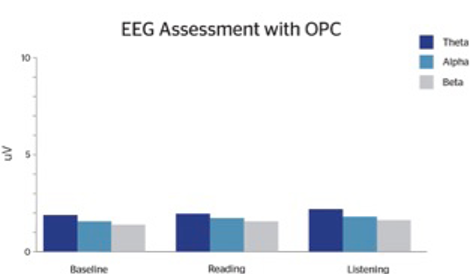
An earlier experiment conducted by psychologist Marion Sigurdson, involving 30 subjects with ADHD, found that the subjects’ attention and concentration improved just as much with a daily regimen of pine-bark-derived OPCs as with traditional ADHD stimulant medications.8 Additionally, the subjects reported experiencing better sleep and improved mood while taking the OPC supplement.
Sigurdson’s experimental outcomes were echoed in later research, including a randomized controlled trial seeking to investigate the effects of pycnogenol on attention, oxidative DNA damage, and antioxidant status in ADHD patients and healthy controls. In a 2006 study, 61 children aged 6-14 with ADHD were randomly assigned to receive either pycnogenol (1 milligram per kilogram of body weight) or a placebo for one month, without any additional medications or supplements. Baseline measures taken at the start of the trial revealed that children with ADHD had significantly more oxidative DNA damage than their non-ADHD counterparts; after the study, markers of DNA damage were significantly lower in the group that had received pycnogenol, even compared to measures taken from the non-ADHD controls.9 At the end of the study, children who had taken pycnogenol had significantly elevated measures of antioxidant status. Within a month of discontinuing the supplement, however, subjects displayed increased levels of DNA damage. The data generated by this randomized trial points to a strong association between DNA oxidation, total antioxidant status, and inattention.
Additional evidence in support of the efficacy of OPCs for ADHD comes from the anecdotal experience of psychologist Dr. Steven Tenenbaum, who was diagnosed with ADHD as a child and lived with the disorder through adulthood. In addition to being a clinical psychologist, Tenenbaum was an aviation enthusiast. Due to FAA regulations, he was unable to utilize pharmaceutical treatments for his ADHD, and the use of stimulant drugs would cost him his pilot’s license. He turned to alternative therapies and, in 1995, initiated a regimen of pycnogenol three times daily. According to Tenenbaum, the results were impressive: with pycnogenol, he reported increased attention, improved focus, decreased emotional volatility, and better mood. Without pycnogenol, Tenenbaum reported that ADHD symptoms would return immediately.
The effects of OPCs have been tested both in ADHD patients as well as healthy, non-ADHD individuals. One study explored the effects of pycnogenol on the cognitive abilities and emotional status of 53 healthy students aged 18-27. The students were tested both before and after a regimen of pycnogenol (100mg/day) in measures of attention, memory, alertness, executive functioning, and mood, and showed significant improvements across the board after eight weeks of pycnogenol supplementation.10
A more recent study out of the University of Exeter documented the positive effects that drinking blueberry juice (rich in OPC flavonols) has upon brain function in older adults.11 In the study, healthy participants age 65-77 were randomly assigned to one of two experimental groups: members of the first group drank 30ml/day of blueberry juice concentrate for 12 weeks, while members of the second were given a placebo. Before and after the 12-week period, participants underwent a battery of cognitive tests while an MRI scanner monitored their brain function and resting brain blood flow. Compared to the placebo group, those who took the blueberry supplement showed improvements in cognitive function, working memory, blood flow to the brain, and brain activation while carrying out cognitive tests. Finally, a noteworthy study out of the UK examined the effects of blueberry extract consumption on cognition in children and found that every measure of mental ability, including memory, improved in those children who drank blueberry extract.12
Neuroprotective Properties of OPCs
There are several proposed mechanisms for age-related cognitive deficit in memory and learning. Animal studies have shown that oxidative stress within aging neurons can be modified with the neuroprotective effects of OPCs. In one study, OPCs decreased free radical damage and increased the activity of enzymes responsible for clearing these reactive-oxygen species among animals with deteriorated memory and learning abilities.13 New insights into ways of protecting the aging brain from memory deficits has led studies into the histological modification of vascular endothelial growth factor receptors (VEGFR) within the brain, a signal protein produced by cells that stimulates vasculogenesis and angiogenesis.14
Moreover, several animal studies have shown the positive benefit of OPCs to affect mood and reduce depressive symptoms.15 In a double blinded, randomized, crossover study, patients with chronic fatigue syndrome had improved depression and anxiety symptoms after eight weeks of OPC rich polyphenol supplements.16 Another cross-sectional study of 42,093 Japanese adults aged greater than 40 years old from the general population reported that green tea was inversely associated with psychological distress even after adjustment for possible confounding factors.17 When solely looking at depressive symptoms, green tea was also associated with a lower prevalence in the Japanese community-dwelling older population.18
Bolstering the Blood-Brain Barrier
The blood-brain barrier (BBB) is a semipermeable barrier between the blood and brain that allows specific substances of a certain size and chemistry to pass through. The physical and functional integrity of the BBB is of paramount importance, as the BBB determines what molecules gain access to the brain itself. A healthy BBB is therefore essential for the regulation of biochemical, neurotransmitter, and micronutrient levels within the brain, as well as for the protection of brain cells against substances carried within the blood that may be potentially damaging (e.g., toxins, pollutants).
Unlike many other antioxidants, OPCs have a strong affinity for collagen-elastin crosslinks present within the tight junctions of the BBB. This property allows OPCs to not only cross the BBB but also protect and maintain regulatory mechanisms present within the BBB. In vivo animal studies have shown that oral administration of OPC can greatly increase the resistance of brain capillaries to the hydrolytic action of bacterial collagenases injected into their lateral ventricles, sustaining the collagen cross-linking component of the basement membrane.19
The BBB also helps maintain physiologic molecules from leaking out of the brain for maintaining a proper homeostatic environment. One study evaluated fourteen boys (ages 6-12 years) with ADHD for deficiency in necessary brain chemicals.20 They found that ADHD patients had nearly 50% lower amino acid levels of tryptophan and much higher levels of alanine than normal brains. Decreased transport of tryptophan due to a dysfunctional blood-brain barrier can lead to a further deficiency in serotonin access in the brain that might cause disturbances in behavior and cognitive performance.
Maintaining the functional abilities of the blood-brain barrier is integral for protecting the brain from the penetrative harmful substances, such as environmental toxins leading to oxidative damage within the brain. The use of synthetic food additives (i.e., artificial coloring), made from petroleum, and preservatives (i.e., sodium benzoate) has increased by 500% over the past five decades. This increased use introduces children to the greatest foreign antigenic load, challenging their immune system.
These dyes are naturally small and therefore able to easily evade the host immune response. Additionally, they are able to either bind body proteins to form immune complexes (Antigen-IgG) that are able to travel through an impaired BBB and deposit within the brain and/or peripherally mediate the release of histamine from mast cells and basophils. A double-blind placebo controlled food challenge was completed on 16 children and showed that on days with ingestion of reactive foods, their symptoms were significantly exacerbated when compared to placebo days.21 Another study on 15 patients suffering from food-induced ADHD employed topographic electroencephalographic (EEG) mapping to show that intake of provoking foods directly increased brain electrical activity in the fronto-temporal areas of the brain.22
Miracle-Gro for Neurons: Brain-Derived Neurotrophic Factor (BDNF)
Multiple research studies have demonstrated that OPCs will enhance the production of brain-derived neurotrophic factor (BDNF). BDNF is a protein found in the central and peripheral nervous system that plays an essential role in the growth, differentiation, and maturation of neurons, as well as developing and maintaining the connections between neurons.23 BDNF is a mediator of neuroplasticity, a term used to describe the brain’s ability to reorganize itself in response to changing patterns of stimulation. BDNF activity is crucial in supporting the brain’s ability to respond to novel demands, such as learning new information, being stimulated in a new way, or even adaptively adjusting to compensate for damage. Experimental studies have shown that consumption of OPC-rich foods such as green tea and dark chocolate increases BDNF levels. Adequate BDNF levels are critical for ADHD patients who might struggle with learning as a result of activational impairments in reward processing centers of the brain, or impaired electrochemical signaling between neural networks secondary to diminished functional connectivity.24
Clinical Utility of OPCs in ADHD Treatment
There is no such thing as a ‘universal fix’ or ‘magic bullet,’ for any major neuropsychiatric illness. Thanks to differences in genetic makeup, DNA expression, nutrition, physical activity, living environment, and patterns of social interactions, each and every individual ADHD patient is unique. What works for one patient may not necessarily work for another, and OPCs may not be the solution for every person with ADHD. However, for many inattentive, distractible children (and adults), OPCs can be efficacious as an adjunct therapy, as we have evaluated the empirical data that confirms OPCs’ ability to dramatically improve clinical symptoms of ADHD while minimizing the use of high-dose stimulant medications.
Science has demonstrated that OPCs directly benefit a host of brain regions, brain networks, systems of neuron-to-neuron signaling, biochemical and metabolic processes, and metabolic ratios that have been identified as being responsible for many of the symptoms of ADHD. There are several proposed theories about how OPCs can minimize symptoms of hyperactivity, improve focus, regulate mood lability, and even combat age-related cognitive decline. As a potent antioxidant, OPCs is involved in several roles that support neurotransmitter activity, neuronal growth, and immunological function. While we do not know the exact mechanism, the available literature supports OPCs as a safe, natural, and therapeutic alternative or adjunct treatment that can improve cognitive performance and minimize ADHD symptoms.
The use of OPC supplements has not only benefited patients with ADHD, but scientists have also found that they can be useful in combating depression and neurodegenerative diseases associated with the aging process by stimulating neuronal growth and enhancing the integrity of the blood-brain barrier.
In two decades of using OPCs to treat patients with ADHD, I have observed countless patients whose thinking becomes progressively clearer once they start taking OPCs. OPCs can be an effective biological alternative in the treatment of adults and children with ADHD. By incorporating them into modern-day nutritional psychiatry, we can offer countless patients relief from symptoms.
References
- Arns M et al. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta‐analysis. Clin EEG Neurosci. 2009;40(3):180‐9.
- Mann C et al. Quantitative analysis of EEG in boys with Attention Deficit‐Hyperactivity Disorder (ADHD): A controlled study with clinical implications. Pediatric Neurology. 1992;8:30‐36.
- Steiner NJ et al. In‐school neurofeedback training for ADHD: Sustained improvements from a randomized control trial. Pediatrics. 2014;133(3): 483‐492
- Duric NS et al. Neurofeedback for the treatment of children and adolescents with ADHD: a randomized and controlled clinical trial using parental reports. BMC Psychiatry. 2012;12: 107.
- González‐Castro P et al. Efficacy of neurofeedback versus pharmacological support in subjects with ADHD. Appl Psychophysiol Biofeedback. 2016;41:17.
- Lamb J. (2011). Captain Cook and the scourge of scurvy. BBC.co.uk. Retrieved from
http://www.bbc.co.uk/history/british/empire_seapower/captaincook_scurvy_01.shtml - Chaudhary AK et al. Cognitive enhancement in aged mice after chronic administration of Cedrus deodara Loud. and Pinus roxburghii Sarg. with demonstrated antioxidant properties. J Nat Med. 2013;68(2):274‐83.
- Carper J. (1998). Miracle cures. New York, NY: HarperPerennial. pp.221‐236.
- Chovanová Z et al. Effect of polyphenolic extract, Pycnogenol, on the level of 8‐oxoguanine in children suffering from attention deficit/hyperactivity disorder. Free Radical Research. 2006;40(9):1003–10.
- Luzzi R et al. Pycnogenol supplementation improves cognitive function, attention and mental performance in students. Panminerva Medica. 2011;53(3‐1):75–82.
- Bowtell JL et al. Enhanced task related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl Physiol Nutr Metab. 2017;1:1‐7.
- Whyte AR et al. Effects of a single dose of a flavonoid‐rich blueberry drink on memory in 8 to 10 y old children. Nutrition. 2015;31(3):531‐4.
- Duan Y et al. The preventive effect of lotus seedpod procyanidins on cognitive impairment and oxidative damage induced by extremely low frequency electromagnetic field exposure. Food Funct. 2013;4(8):1252‐62.
- Yokozawa T et al. Role of oligomeric Proanthocyanidins derived from an extract of persimmon fruits in the oxidative stress‐related aging process. Molecules. 2014;19(5):6707‐26.
- Messaoudi M et al. Antidepressant‐like effects of a cocoa polyphenolic extract in Wistar‐Unilever rats. Nutr Neurosci. 2008;11(6):269‐76.
- Sathyapalan T et al. High cocoa polyphenol rich chocolate may reduce the burden of symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55.
- Hozawa A et al. Green tea consumption is associated with lower psychological distress in a general population: the Ohsaki Cohort 2006 Study. Am J Clin Nutr. 2009;90:1390‐6.
- Niu K et al. Green tea consumption is associated with depressive symptoms in the elderly. Am J Clin Nutr. 2009;90:1615‐22.
- Robert A et al. Effect of procyanidolic oligomers on the permeability of the blood‐brain barrier. Pathologie Biologie. 2001;49(4):298‐304.
- Johansson J et al. Altered tryptophan and alanine transport in fibroblasts from boys with attention‐deficit/hyperactivity disorder (ADHD):an in vitro study. Behav Brain Func. 2011;24(7):40.
- Boris M et al. Foods and additives are common causes of the attention deficit hyperactive disorder in children. Ann Allergy. 1994;72(5):462‐8.
- Uhlig T et al. Topographic mapping of brain electrical activity in children with food‐induced attention deficit hyperkinetic disorder. Eur J. Pediatr. 1997;156(7):557‐61.
- Karege F et al. Decreased serum brain‐derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143‐8.
- Takeda A et al. Facilitated neurogenesis in the developing hippocampus after intake of theanine, an amino acid in tea leaves, and object recognition memory. Cellular and Molecular Neurobiology. 2011;31(7):1079–88.